Abstract
Introduction: Recombinant von Willebrand factor (rVWF) has demonstrated hemostatic efficacy and safety when used for on-demand treatment and for control of bleeding episodes in the surgical setting in patients with severe von Willebrand disease (VWD). rVWF is administered without recombinant FVIII (rFVIII) if an immediate rise in FVIII coagulant activity (FVIII:C) is not necessary or if baseline FVIII:C is sufficient to ensure hemostasis (≥40-100 IU/dL). In the elective surgical setting, rVWF may be given alone prior to surgery to raise endogenous FVIII:C. We analyzed prospective clinical trial data to better inform dosing decisions related to treatment and the pharmacodynamic effect of administering rVWF on endogenous FVIII:C.
Methods: Three clinical trials were included in this post-hoc analysis: a phase 3, on-demand study (NCT01410227), a phase 3, elective surgery study (NCT02283268), and a phase 1 study (NCT00816660; included only in correlation analyses). All patients had severe VWD (VWF ristocetin cofactor activity [RCo] <20 IU/dL for type 1, multimer pattern for type 2A, genotype for type 2B, FVIII:C <10 IU/dL with historically documented genetics for type 2N, VWF antigen ≤3 IU/dL for type 3). Patients who received rVWF alone at doses of 50 or 80 IU/kg VWF:RCo were included in the analysis of mean endogenous FVIII:C over time and the proportion of patients with FVIII:C >40 IU/dL and >60 IU/dL at specific time points. These analyses were based on blood samples obtained at multiple times during the studies. Patients who received rVWF at doses of 7.5, 20, 50, or 80 IU/kg VWF:RCo, alone or with rFVIII (Antihemophilic Factor [Recombinant]), were included in the analysis of the correlation between incremental recovery (IR) at Cmax (VWF:RCo) and body mass index (BMI). Pharmacokinetic assessments were performed at multiple time points before and after infusion of rVWF.
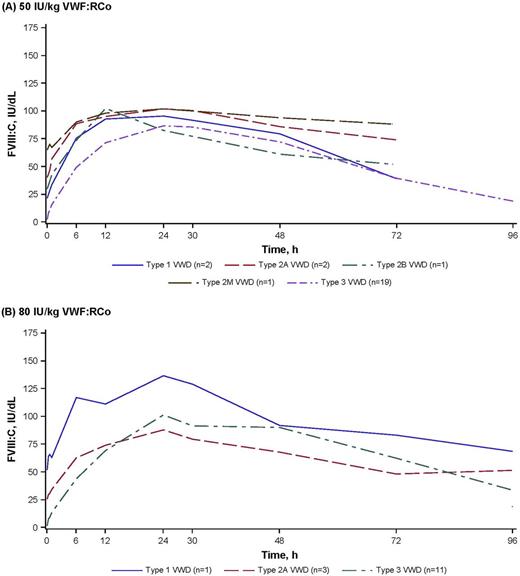
Results: This analysis included 25 patients (12 female/13 male; 19/25 with type 3 VWD) treated with rVWF 50 IU/kg VWF:RCo and 15 patients (9 female/6 male; 11/15 with type 3 VWD) treated with 80 IU/kg VWF:RCo. Treatment with rVWF alone resulted in rapid and sustained increases in endogenous FVIII:C, regardless of the type of VWD (Figure). Among 36 patients with a preinfusion FVIII:C level of <40 IU/dL, the mean time to reach 40 IU/dL FVIII:C was 5.1 h (range: 0.8-11.5) in all patients and 5.6 h (range: 1.6-11.5) in patients with type 3 VWD. Patients with type 1, 2A, and 2B seem to have achieved the 40-IU/dL level faster (1.5, 4.2, and 0.8 h, respectively). Mean FVIII:C levels were 18.8 IU/dL (range: 4.0-69.8) as early as 30 min postinfusion, 56.6 IU/dL (range: 8.0-116.8) 6 h after infusion, and 98.2 IU/dL (range: 51.9-177.7) 24 h after infusion. Corresponding levels in patients with type 3 VWD were 10.9 IU/dL (range: 4.0-24.1) 30 min postinfusion, 48.6 IU/dL (range: 8.0-108.3) 6 h after infusion, and peaked at 103.8 IU/dL (range: 53.0-178.0) after a mean of 29.1 h (11.0-48.1) postinfusion. The mean difference between the pre-infusion FVIII:C and the 6-h FVIII:C for both 50 IU/kg and 80 IU/kg was 45.9 IU/dL (range: 6.0-103.3). At 6 h after administration of rVWF alone, 70% (28/40) of all patients and 63.3% (19/30) of patients with type 3 VWD had FVIII:C >40 IU/dL and 47.5% (19/40) and 33.3% (10/30), respectively, had FVIII:C >60 IU/dL. Lastly, BMI was a predictor of VWF:RCo recovery, suggesting that ideal body weight rather than actual body weight be used in dose calculations.
Conclusions: Treatment with rVWF alone results in rapid stabilization of endogenous FVIII:C, which was more rapid in patients with higher baseline FVIII:C levels (eg, type 1 and type 2 VWD). FVIII:C increases at a mean rate of 7.7 IU/dL per hour (range: 1.0-17.2). Therefore, a rise in FVIII:C to hemostatically effective levels (≥40 IU/dL) was reached in the majority of patients within 6 h, and sooner in patients with higher FVIII:C levels at baseline. These data may help physicians decide whether and when exogenous rFVIII is required at the first dose of rVWF, and they provide additional information on the timing of the endogenous FVIII rise in patients with different VWD types. rVWF dosing should be based on ideal body weight particularly for patients who are underweight or overweight.
Figure. Mean FVIII stabilization for patients with severe VWD who received rVWF alone
Gill: Shire: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Ragni: A Anylam, Biomarin, Bioverativ, Shire: Honoraria; Alnylam, CSL Behring, BAYER, Biomarin, Biomarin, Bioverativ, Genetech/Roche, Pfizer, Shire, SPARK: Research Funding. Castaman: Shire: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees; Kedrion: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees. Peyvandi: Freeline, Kedrion, LFB, Octapharma: Consultancy; Ablynx, Bayer, Grifols, Novo Nordisk, Sobi: Speakers Bureau; Ablynx, Roche: Membership on an entity's Board of Directors or advisory committees. Ploder: Shire: Employment. Novack: Shire: Employment, Equity Ownership. Chen: Shire: Employment. Sytkowski: Shire: Employment, Equity Ownership. Ewenstein: Shire: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal